The vaccine research and development community has faced unprecedented challenges in recent months, including policy upheavals, leadership changes, research program cancellations, and a surge of misinformation have created uncertainty and slowed progress. For many scientists and innovators focusing on vaccines, this has been a difficult time.
At Polygon Health Analytics, we believe that access to reliable data should never be a barrier to scientific progress. To address these challenges, we’re proud to introduce the Vaccine Trial Atlas, a free, interactive dashboard designed to make vaccine-related clinical trial data easier to find, understand, and use.
Why Vaccine Trial Atlas?
ClinicalTrials.gov, launched in 2000, remains the cornerstone for clinical trial transparency. However, its complexity often limits usability for researchers, healthcare professionals, policymakers, and the public. Common challenges include:
- A non-intuitive interface and overwhelming search experience
- Dense, technical language in trial descriptions
- Inconsistent formatting and terminology across studies
- Limited filtering options for targeted analysis
These barriers make it difficult, even for experts, to extract actionable insights.
Our Solution
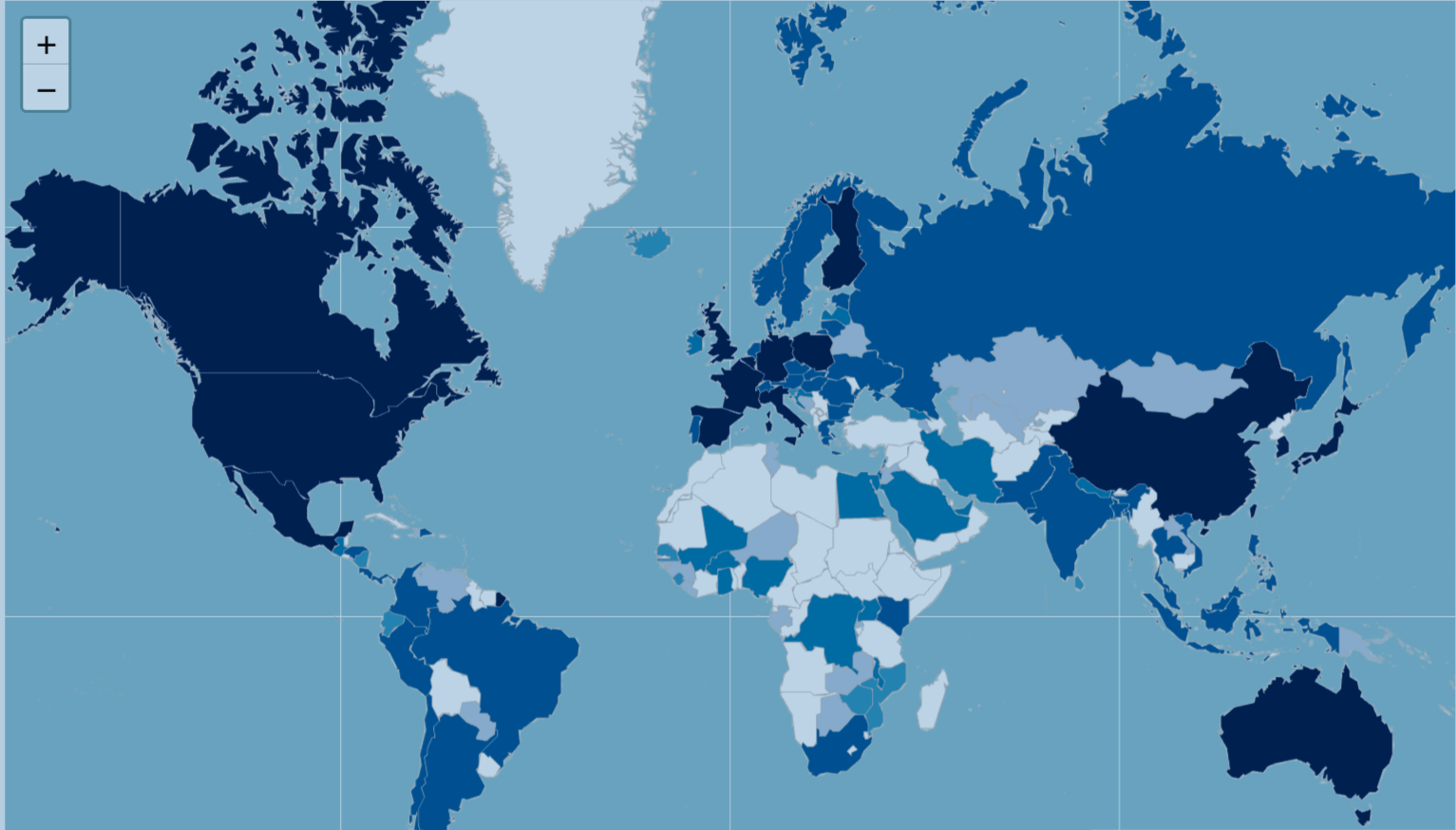
The Vaccine Trial Atlas simplifies this process by providing a centralized, user-friendly platform focused on interventional vaccine studies. Built on data from the Aggregate Analysis of ClinicalTrials.gov (AACT), the dashboard offers:
- Comprehensive Data Access – Aggregated vaccine trial data with advanced filtering
- Standardized Terminology – Consistent mapping of conditions and interventions
- Customizable Search & Filters – Explore by disease, phase, geography, sponsor type, and more
- Interactive Visualizations – Track trends across time, regions, and trial phases
- Export & Share – Download datasets in CSV for deeper analysis and collaboration
👉 [Explore the Vaccine Trial Atlas]
Join Us in Driving Transparency
The Vaccine Trial Atlas is just the beginning. We invite researchers, organizations, and policymakers to collaborate with us as we expand this platform to cover broader therapeutic areas and more advanced features.
For feedback, inquiries, or partnership opportunities, contact us at info@polygonhealthanalytics.com.